
 i_need_contribute
i_need_contribute

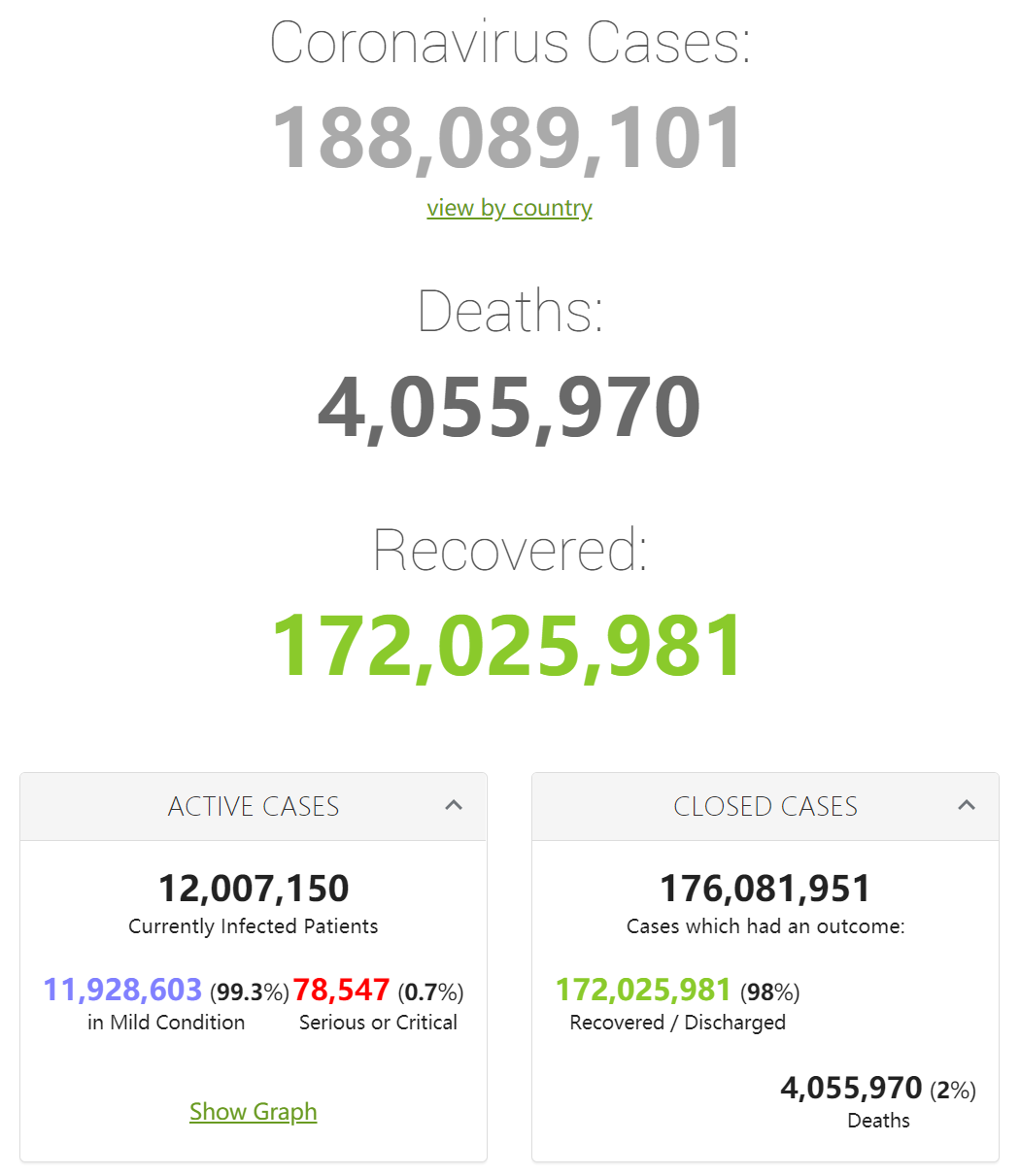
|
Country, |
Total |
New |
Total |
|
World |
188,057,208 |
+391,897 |
4,055,634 |
|
34,766,404 |
+14,715 |
623,029 |
|
|
30,904,734 |
+30,827 |
409,338 |
|
|
19,106,971 |
+17,031 |
534,311 |
|
|
5,813,899 |
+1,260 |
111,353 |
|
|
5,808,473 |
+25,140 |
143,712 |
|
|
5,486,959 |
+5,404 |
50,278 |
|
|
5,155,243 |
+34,471 |
128,431 |
|
|
4,662,937 |
+14,989 |
99,255 |
|
|
4,530,610 |
+18,650 |
113,335 |
|
|
4,272,163 |
+888 |
127,788 |
|
|
3,971,124 |
+11,310 |
81,020 |
|
|
3,744,267 |
+554 |
91,774 |
|
|
3,394,279 |
+20,829 |
86,041 |
|
|
2,880,865 |
+44 |
75,160 |
|
|
2,590,500 |
+3,779 |
234,969 |
|
|
2,567,630 |
+40,427 |
67,355 |
|
|
2,241,217 |
+174 |
52,604 |
|
|
2,206,781 |
+11,182 |
64,509 |
|
|
2,081,557 |
+780 |
194,488 |
|
|
1,736,879 |
+8,441 |
17,766 |
|
|
1,669,506 |
+148 |
30,333 |
|
|
1,589,623 |
+2,145 |
33,980 |
|
|
1,478,061 |
+5,204 |
26,015 |
|
|
1,438,511 |
+9,149 |
17,592 |
|
|
1,421,108 |
+577 |
26,438 |
|
|
1,096,134 |
+1,086 |
25,204 |
|
|
1,081,275 |
+39 |
34,219 |
|
|
1,034,957 |
+13,768 |
16,639 |
|
|
975,092 |
+1,808 |
22,597 |
|
|
909,756 |
+1,782 |
17,164 |
|
|
846,327 |
+193 |
6,438 |
|
|
844,870 |
+8,574 |
6,260 |
|
|
820,715 |
+2,032 |
14,955 |
|
|
808,539 |
+102 |
30,007 |
|
|
757,690 |
+767 |
9,843 |
|
|
717,667 |
+118 |
7,073 |
|
|
657,139 |
+1,690 |
9,400 |
|
|
651,804 |
+192 |
10,723 |
|
|
651,762 |
+1,542 |
1,870 |
|
|
547,961 |
+150 |
7,877 |
|
|
543,119 |
+657 |
9,384 |
|
|
502,439 |
+1,244 |
7,992 |
|
|
501,923 |
+4,310 |
16,494 |
|
|
468,414 |
+68 |
21,830 |
|
|
458,724 |
+3,899 |
4,716 |
|
|
454,241 |
+646 |
17,219 |
|
|
440,872 |
+2,063 |
12,778 |
|
|
438,764 |
+1,045 |
13,964 |
|
|
428,595 |
+689 |
3,266 |
|
|
422,545 |
+83 |
18,144 |
|
|
416,232 |
+752 |
6,646 |
|
|
391,925 |
+7 |
12,521 |
|
|
383,500 |
+785 |
4,812 |
|
|
377,811 |
+659 |
5,468 |
|
|
377,364 |
+1,770 |
2,136 |
|
|
376,876 |
+297 |
5,833 |
|
|
360,841 |
+15 |
8,229 |
|
|
345,027 |
+8,656 |
2,791 |
|
|
337,323 |
+69 |
4,987 |
|
|
319,157 |
+616 |
9,721 |
|
|
315,224 |
+64 |
3,582 |
|
|
300,071 |
+848 |
2,539 |
|
|
286,072 |
+1,167 |
3,435 |
|
|
285,910 |
+968 |
3,302 |
|
|
283,212 |
+110 |
16,403 |
|
|
279,216 |
+21 |
4,401 |
|
|
278,464 |
+572 |
5,006 |
|
|
277,137 |
+66 |
4,343 |
|
|
276,106 |
+1,568 |
3,533 |
|
|
274,478 |
+776 |
7,259 |
|
|
267,324 |
+112 |
1,378 |
|
|
257,875 |
+12 |
4,425 |
|
|
257,529 |
+62 |
6,211 |
|
|
244,914 |
+6,423 |
1,579 |
|
|
226,459 |
+71 |
4,547 |
|
|
223,504 |
+146 |
599 |
|
|
206,769 |
+2,679 |
3,243 |
|
|
197,227 |
+5,014 |
3,927 |
|
|
188,942 |
+188 |
3,723 |
|
|
176,742 |
+1,013 |
2,867 |
|
|
169,146 |
+1,100 |
2,044 |
|
|
168,713 |
+161 |
2,124 |
|
|
146,942 |
+878 |
3,851 |
|
|
140,978 |
+1,330 |
2,113 |
|
|
139,284 |
+1,496 |
689 |
|
|
137,917 |
+18 |
2,536 |
|
|
133,367 |
+170 |
796 |
|
|
132,597 |
+5 |
2,456 |
|
|
131,508 |
+21 |
1,270 |
|
|
116,421 |
+458 |
774 |
|
|
106,879 |
+1,284 |
2,019 |
|
|
100,488 |
+10 |
1,621 |
|
|
98,215 |
+133 |
976 |
|
|
92,066 |
+27 |
4,636 |
|
|
90,555 |
+1,687 |
996 |
|
|
87,756 |
+479 |
2,129 |
|
|
85,104 |
+832 |
380 |
|
|
32,199 |
+2,383 |
125 |
|
|
23,463 |
+80 |
587 |
Retrieved from: https://www.worldometers.info/coronavirus/
Reuters
The World Health Organization's chief scientist on Monday advised against people mixing and matching COVID-19 vaccines from different manufacturers, calling it a "dangerous trend" since there was little data available about the health impact.
"It's a little bit of a dangerous trend here. We are in a data-free, evidence-free zone as far as mix and match," Soumya Swaminathan told an online briefing.
"It will be a chaotic situation in countries if citizens start deciding when and who will be taking a second, a third and a fourth dose."
Retrieved from: https://www.reuters.com/business/healthcare-pharmaceuticals/who-warns-against-mixing-matching-covid-vaccines-2021-07-12/
By Michael Erman
The U.S. Food and Drug Administration on Monday added a warning to the fact sheet for Johnson & Johnson's (JNJ.N) COVID-19 vaccine saying that data suggests there is an increased risk of a rare neurological disorder in the six weeks after inoculation.
In a letter to the company, the FDA classified the chances of getting Guillain-Barré syndrome (GBS) after vaccination as being "very low." Still, it said J&J vaccine recipients should seek medical attention if they have symptoms including weakness or tingling sensations, difficulty walking or difficulty with facial movements.
Around 12.8 million people have received J&J's one-dose vaccine in the United States. The FDA said 100 preliminary reports of GBS in the vaccine recipients include 95 serious cases that required hospitalization and one reported death.
J&J said in a statement that it was in discussion with regulators about the cases of GBS. It said the rate of reported cases of GBS in J&J vaccine recipients exceeds the background rate only by a small degree.
GBS is a rare neurological condition in which the body's immune system attacks the protective coating on nerve fibers. Most cases follow a bacterial or viral infection. Most people fully recover from GBS.
The condition has been linked in the past to vaccinations - most notably to a vaccination campaign during a swine flu outbreak in the United States in 1976, and decades later to the vaccine used during the 2009 H1N1 flu pandemic.

A box of Johnson & Johnson's coronavirus disease (COVID-19) vaccines is seen at the Forem vaccination centre in Pamplona, Spain, April 22, 2021. REUTERS/Vincent West/File Photo
According to a statement from the U.S. Centers for Disease Control and Prevention (CDC), most of the cases were in men, many of whom were 50 or older. It did not find higher than expected cases of GBS in recipients of the mRNA-based vaccines from Pfizer Inc (PFE.N)/BioNTech SE and Moderna Inc. (MRNA.O)
Last week, European regulators recommended a similar warning for AstraZeneca's COVID-19 shot, which is based on a similar technology as Johnson & Johnson's vaccine.
The warning is another setback for the J&J shot, which was supposed to be an important tool for vaccinating in hard-to-reach areas and among those hesitant to be vaccinated because it requires only one shot and has less stringent storage requirements than the Pfizer or Moderna vaccines.
But use of the vaccine has already been linked to a very rare, potentially life threatening blood clotting condition and slowed by production problems at the main plant where it is being made.
U.S. regulators decided in April that the vaccine's benefits outweighed the risk from the blood clotting issue.
The warning was first reported by the Washington Post on Monday.
Retrieved from: https://www.reuters.com/business/healthcare-pharmaceuticals/us-announce-new-warning-jj-coronavirus-vaccine-autoimmune-disorder-washington-2021-07-12/

Workers unloaded boxes containing the Janssen vaccine in Kathmandu, Nepal, on Monday.Credit...Narendra Shrestha/EPA, via Shutterstock
The Gavi Alliance announced Monday that it had signed its first agreements to buy coronavirus vaccine from two Chinese companies, Sinopharm and Sinovac, providing for deliveries of 110 million doses within three months, with options for bigger deliveries later this year and in the first half of 2022.
Gavi, the public-private partnership that is overseeing Covax, the program to donate vaccines to poor countries, said that Sinopharm would contribute 60 million doses between July and October, with an option to provide 60 million more in the last quarter of 2021 and 50 million more doses in the first half of 2022.
Sinovac would deliver 50 million doses, Gavi said, by the end of September, with an option for Covax to receive 150 million more doses in the last quarter of the year and 180 million doses in the first half of 2022.
The agreements, combined with donations pledged by the United States and other Group of 7 countries, will give a boost to Covax. It was set up to help the poorest countries gain access to vaccines, but it has struggled to gain a footing, as world leaders and vaccine manufacturers have prioritized sending doses to populations in wealthy nations.
China kicked off its vaccine diplomacy campaign last year by pledging to provide a shot that would be safe and effective at preventing severe cases of Covid-19, and dozens of countries are using Covid vaccines from China. Some of the countries that have experienced fresh virus outbreaks despite high inoculation rates, though, relied on Chinese-made vaccines. During a news conference Monday, World Health Organization officials supported the addition of the Sinovac and Sinopharm vaccines to the Covax portfolio, and expressed confidence in the efficacy of the vaccines.
But as concerns grow about more transmissible variants as well as about the waning immunity provided by the Sinovac vaccine, Thailand said on Monday that health care workers who had received the vaccine made by Sinovac would also be inoculated with the Oxford-AstraZeneca or Pfizer-BioNTech shots to give them greater protection.
About 3.4 billion Covid-19 vaccine doses have been administered globally but the vast majority have gone to wealthy countries. Covax, since its launch in February, has shipped 107 million vaccine doses to 135 countries. About 70 percent of these doses have gone to poorer countries; the other 30 percent have gone to wealthier nations paying their own way.
Gavi said that it is now on track to deliver nearly 1.9 billion doses in 2021. It said that it will reach its target of two billion doses distributed in early 2022, instead of by the end of this year.
Covax supplies only W.H.O.-approved vaccines, and Gavi noted that it had approved an emergency-use listing for Sinopharm in May and for Sinovac in June, clearing the way for rapid delivery of the vaccines.
Covax signed an agreement with a third Chinese vaccine manufacturer, Clover, at the end of June, for a total of 414 million doses. Covax said that 64 million doses would be available in the last quarter of the year. Clover, however, is still awaiting W.H.O. emergency-use listing, creating uncertainty over when Covax will be able to start delivering the vaccine.
By Sheri Fink

ECMO, short for extracorporeal membrane oxygenation, adds oxygen and removes carbon dioxide from a patient’s blood before pumping it back in.Credit...Victor J. Blue for The New York Times
Throughout the pandemic, wrenching scenes have played out across the United States as doctors found themselves in the unfamiliar position of overtly rationing a treatment. But it was not ventilators, as initially feared: Concerted action largely headed off those shortages. Instead, it was the limited availability of ECMO — which requires expensive equipment similar in concept to a heart-lung machine and specially trained staff who can provide constant monitoring and one-on-one nursing — that forced stark choices among patients.
“Patients died because they could not get ECMO,” said Dr. Lena M. Napolitano, co-director of the Surgical Critical Care Unit at the University of Michigan. This spring, she was overwhelmed with requests to accept patients considered good candidates for ECMO. “We could not accommodate all of them,” she said.
Doctors tried to select individuals most likely to benefit from ECMO, a last-resort treatment that can mechanically substitute for badly damaged lungs. But dozens of interviews with medical staff and patients across the country, and reporting inside five hospitals that provide ECMO, revealed that in the absence of regional sharing systems to ensure fairness and match resources to needs, hospitals and clinicians were left to apply differing criteria, with insurance coverage, geography and even personal appeals having an influence.
“It’s unsettling to have to make those kinds of decisions,” said Dr. Ryan Barbaro, a critical care physician in Michigan and head of an international registry of Covid-19 patients who have received ECMO — short for extracorporeal membrane oxygenation — about half of whom survived hospitalization.
Close to 8,000 patients worldwide have received ECMO to date, including nearly 5,000 in North America. Despite the progress the United States has made against the coronavirus, some doctors are still having to ration ECMO, which is offered in less than 10 percent of hospitals.
“It’s something we’re balancing every day,” said Dr. Erik Eddie Suarez, a cardiovascular surgeon at Houston Methodist. If the hospital accepts too many Covid patients for ECMO, he said, “we can’t do cardiac surgery,” because some of those patients also need the treatment.

Patients waiting to receive a third dose of the Pfizer-BioNTech coronavirus vaccine on Monday at the outpatient clinics of the Cardiovascular Centre at Sheba Medical Center near Tel Aviv, Israel.Credit...Jack Guez/Agence France-Presse — Getty Images
Israel’s Ministry of Health on Monday issued guidelines for administering a third shot of the two-dose Pfizer-BioNTech coronavirus vaccine to people with compromised immune systems, citing the rising infection rate in recent weeks as well as growing evidence that such people do not develop sufficient antibodies after two doses.
The ministry released a list of those now eligible for a third shot, prioritizing heart, lung and kidney transplant recipients followed by others with weak immune systems including cancer patients.
Sheba Medical Center near Tel Aviv began giving third Pfizer shots to dozens of heart transplant recipients on Monday afternoon, an hour after receiving a green light from the Ministry of Health.
“It’s really urgent to do it now,” Prof. Galia Rahav, the head of the Infectious Disease Unit and Laboratories at the Sheba Medical Center, said in a video statement, citing the rise of the Delta variant. The hospital said it would be testing and tracking the recipients of the third shot for research purposes.
Israel initially led the world with a rapid vaccination campaign and 57 percent of its population is fully vaccinated. But the arrival of the highly contagious Delta variant has brought a rise in daily infections, up from single digits a month ago to an average of 452 cases per day. About 58 percent of the 81 Israeli Covid-19 patients currently hospitalized are vaccinated, according to Israeli Ministry of Health data. Studies suggest that vaccines remain effective against the Delta variant.
Health care providers in France have been giving a third dose of a two-dose vaccine to people with certain immune conditions since April.
The number of organ transplant recipients who had antibodies increased to 68 percent four weeks after the third dose, up from 40 percent after the second dose, one team of French researchers recently reported. In the United States, there has been no concerted effort by federal agencies or vaccine manufacturers to test this approach so far.
Retrieved from: https://www.nytimes.com/2021/07/12/world/middleeast/israel-third-covid-vaccine.html?smid=url-share-live
By Stephen Castle and Mark Landler

Prime Minister Boris Johnson said England will lift coronavirus restrictions starting July 19 as planned. Cases in England continue to rise and the highly contagious delta variant is more widespread.CreditCredit...Jeremie Souteyrat for The New York Times
With coronavirus infections surging yet again, Prime Minister Boris Johnson on Monday urged Britons to keep wearing face masks in crowded, indoor spaces even as he promised to unlock England’s economy next week and lift almost all virus-related restrictions.
Mr. Johnson’s admonition on masks, while not compulsory, represents the latest swerve from a government that delayed the imposition of several lockdowns and then promised the “irreversible” lifting of restrictions, culminating in what British tabloid newspapers called “freedom day.”
Having delayed that moment once, Mr. Johnson on Monday confirmed plans to proceed with the removal of most legal curbs in England on July 19, allowing pubs and restaurants to operate at full capacity and nightclubs to open their doors. Curbs on the number of people who can meet indoors, generally limited to six, will also be lifted.
Despite the spread of the highly transmissible Delta variant, the government believes that Britain’s successful vaccination program has weakened the link between cases and hospital admissions. The government now argues that there is no better time to end lockdown restrictions than in the summer when the virus tends to spread more slowly and schools take a vacation break, eliminating one source of transmission.
Still, the landmark once hailed boldly as “freedom day” by libertarian lawmakers is now being given much more cautious billing by the government as Britain records around 30,000 cases a day, a number that the health secretary, Sajid Javid, on Monday said could climb to 100,000 during the summer.
“Whether we like it or not, coronavirus is not going away,” Mr. Javid said in Parliament.
The government decision to recommend the continued use of face masks in crowded indoor spaces is a shift, in tone at least, from a week ago when Mr. Johnson outlined his thinking at a news conference.
When asked then whether he would wear a mask, he said, “it would depend on the circumstances,” before clarifying later that he would wear one on a crowded train.
On Monday, Mr. Johnson struck a decidedly cautious tone. “I cannot say this powerfully or emphatically enough,” he said at a news conference. “This pandemic is not over. This disease, coronavirus, continues to carry risks for you and your family. We cannot simply revert instantly from Monday, 19 July to life as it was before Covid.”
Mr. Johnson added that the government strongly recommended that people wear a face covering in crowded and enclosed spaces such as on public transportation.
The government plans to work with organizers of large indoor events to encourage the use of certification for those who have been vaccinated or recently tested.
Mr. Johnson said he wanted a gradual return to the workplace rather than a mass move back to offices next week. And Britain’s border restrictions would remain in place, including hotel quarantine for those arriving from countries deemed to be in the highest risk category.
Nonetheless, Mr. Johnson argued that delaying the full reopening of the economy would merely postpone any surge in infections to the fall, when schools return and colder weather gives the virus a natural advantage. So he wants to replace an era of government diktat with one of growing personal responsibility as people learn to live with the virus and use common sense to protect themselves.
Retrieved from: https://www.nytimes.com/live/2021/07/12/world/covid-coronavirus-updates/england-covid-rules-freedom-day?smid=url-share-live
By Sheryl Gay Stolberg and Sharon LaFraniere

Representatives of Pfizer met privately with senior U.S. scientists and regulators on Monday to press their case for swift authorization of coronavirus booster vaccines, amid growing public confusion about whether they will be needed and pushback from federal health officials who say the extra doses are not necessary now.
The high-level online meeting, which lasted an hour and involved Pfizer’s chief scientific officer briefing virtually every top doctor in the federal government, came on the same day Israel started administering third doses of the Pfizer-BioNTech vaccine to heart transplant patients and others with compromised immune systems. Officials said after the meeting that more data — and possibly several more months — would be needed before regulators could determine whether booster shots were necessary.
The twin developments underscored the intensifying debate about whether booster shots are needed in the United States, at what point and for whom. Many American experts, including Dr. Anthony S. Fauci, President Biden’s chief medical adviser for the pandemic, have said there is insufficient evidence yet that boosters are necessary. Some, though, say Israel’s move may foreshadow a government decision to at least recommend them for the vulnerable.
Pfizer is gathering information on antibody responses in those who receive a third dose, as well as data from Israel, and expects to submit at least some of that to the Food and Drug Administration in the coming weeks in a formal request to broaden the emergency authorization for its coronavirus vaccine.
But the final decision on booster shots, several officials said after the meeting, will also depend on real-world information gathered by the Centers for Disease Control and Prevention about breakthrough infections — those occurring in vaccinated people — that cause serious disease or hospitalization.
And any recommendations about booster shots are likely to be calibrated, even within age groups, officials said. For example, if booster shots are recommended, they might go first to nursing home residents who received their vaccines in late 2020 or early 2021, while elderly people who received their first shots in the spring might have a longer wait. And then there is the question of what kind of booster: a third dose of the original vaccine, or perhaps a shot tailored to the highly infectious Delta variant, which is surging in the United States.
“It was an interesting meeting. They shared their data. There wasn’t anything resembling a decision,” Dr. Fauci said in a brief interview Monday evening, adding, “This is just one piece of a much bigger puzzle, and it’s one part of the data, so there isn’t a question of a convincing case one way or the other.”
A spokeswoman for Pfizer said in a statement, “We had a productive meeting with U.S. public health officials on the elements of our research program and the preliminary booster data.”
With less than half of the United States population fully vaccinated, some experts said Monday that the country needed to remain focused on getting all Americans their first dose. The Food and Drug Administration’s most important task, they said, is to increase public confidence by granting full approval to the coronavirus vaccines in use, which for now are authorized on an emergency basis.
“At this point, the most important booster we need is to get people vaccinated,” said Dr. Carlos del Rio, an infectious disease expert at Emory University in Atlanta. The booster doses in Israel, he added, “will help us answer some questions, but at the end of the day I don’t agree with what they’re doing. I think it’s awfully premature.”
Within the Biden administration, some fear that if Americans are convinced that coronavirus vaccines provide only short-lived immunity before requiring a booster, they will be less likely to accept a shot. But those concerns could fall by the wayside if new data from Israel, expected in the next several weeks, shows conclusively that immunity wanes after six to eight months, significantly raising the risks for the elderly or other vulnerable populations.
The administration convened Monday’s session in response to last week’s announcement by Pfizer and its German partner, BioNTech, that they were developing a version of their vaccine that targets the Delta variant, and reporting promising results from studies of people who received a third dose of the original vaccine six months after the second.
The new data is not yet published or peer reviewed, but when the companies announced that they would submit data to the Food and Drug Administration for authorization of booster shots, it caught the Biden White House by surprise.
In an unusual joint statement Thursday evening, hours after the Pfizer-BioNTech announcement, the F.D.A. and the C.D.C. pushed back.
Retrieved from: https://www.nytimes.com/live/2021/07/12/world/covid-coronavirus-updates/pfizer-booster-vaccine-fda-cdc?smid=url-share-live
Here are the key developments from the last few hours:
· France will not allow health workers to go to work and will not pay them if they are not vaccinated against Covid-19 by September 15, the health minister Olivier Veran said.
· Indonesia has reported its highest daily number of infections yet, with 40,427 cases logged on Monday, data from the country’s Covid-19 task force showed. Meanwhile, authorities have expelled four foreign tourists from Bali after they breached the island’s tough coronavirus restrictions.
· South African president Cyril Ramaphosa said that days of protests, looting and riots in the country led to the cancellation of coronavirus vaccination efforts in some parts of the country and could lead to further disruption of the programme just when the country was picking up the pace to inoculate its citizens.
· Vietnam has reported another new record in daily coronavirus infections, with 2,367 cases, its health ministry said.
· Another grim record has been set in Bangladesh, where 13,768 new infections were logged in the 24 hours to Monday morning. A further 220 deaths were also registered.
· The reopening of schools cannot wait for all pupils and teachers to be vaccinated, or for the number of Covid cases to be reduced to zero, the chiefs of Unicef and Unesco have said in a joint statement.
· Dutch prime minister Mark Rutte apologised for relaxing coronavirus restrictions too soon as cases surge in the wake of reopening.
· The number of people who did not have enough food to eat rose steeply during the Covid-19 pandemic to include almost a third of the world, according to a new UN report published on Monday.
· Valencia’s regional government has succeeded in obtaining a court order to authorise lockdowns in more than 30 towns in eastern Spain as cases surge among unvaccinated young people.
· Healthcare workers and nursing home staff in Greece will be required to be vaccinated against Covid, prime minister Kyriakos Mitsotakis has said as infections rapidly soar again after a sustained decline.
Retrieved from: https://www.theguardian.com/world/live/2021/jul/12/coronavirus-live-news-england-toughest-curbs-in-force-in-south-korea-capital-covid