
 i_need_contribute
i_need_contribute

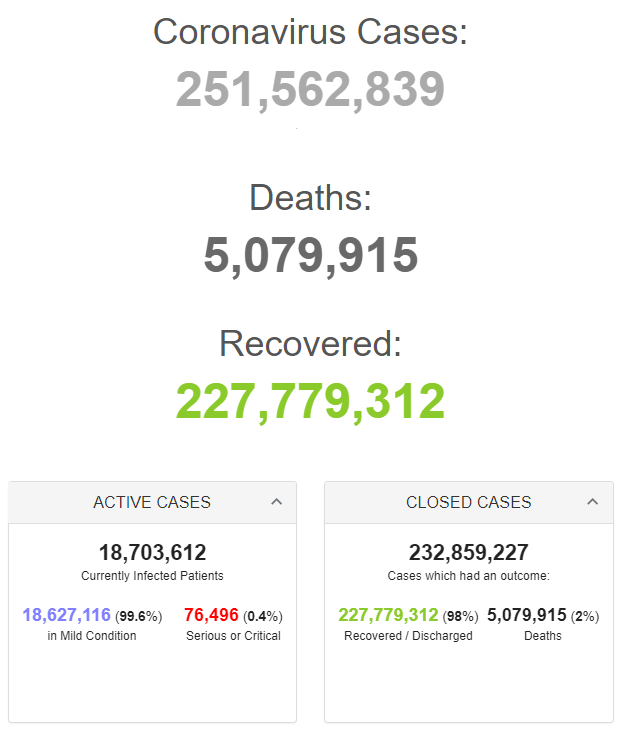
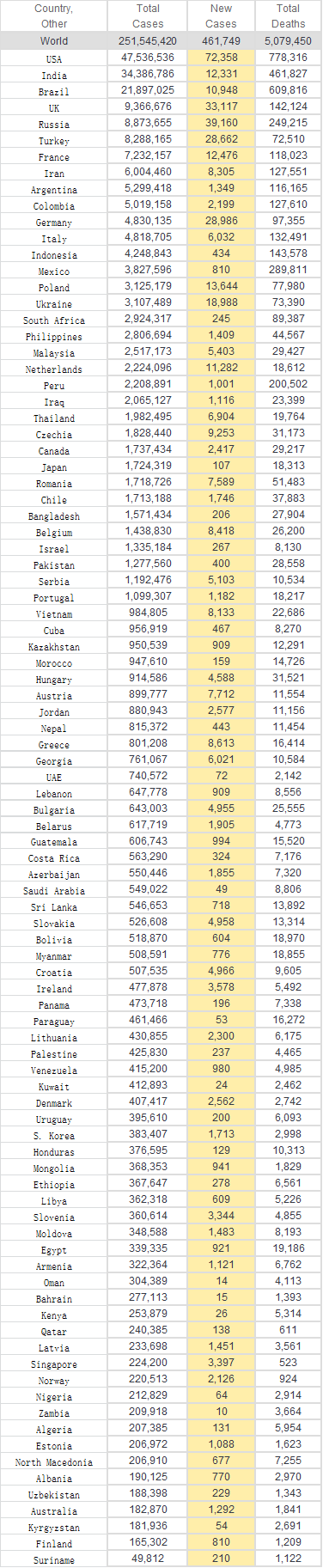
Retrieved from: https://www.worldometers.info/coronavirus/
A medical worker fills a syringe with a dose of the Pfizer-BioNTech coronavirus disease (COVID-19) vaccine as Japan launches its inoculation campaign, at Tokyo Medical Center in Tokyo, Japan February 17, 2021. Behrouz Mehri/Pool via REUTERS/File Photo
Pfizer Inc (PFE.N) has applied for approval from Japan's health ministry to use COVID-19 vaccines on children aged 5 to 11, it said in a statement released on Wednesday.
Pfizer's Comirnaty vaccine, developed in partnership with BioNTech (22UAy.DE), was the first COVID-19 vaccine approved for Japan's general public in February. COVID-19 vaccines developed by Moderna and AstraZeneca have since been approved.
Pfizer's vaccine is currently being offered to those aged 12 and above. If approved by the health ministry, it will be the first to be administered to children under 11 in Japan.
About 74% of the population is now fully vaccinated, according to broadcaster NHK, and the government is widely expected to start administering booster shots by year-end.
Retrieved from: https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-applies-covid-19-vaccine-approval-5-11-year-olds-japan-2021-11-10/
By Tom Hals

A banner asking patients to wear a mask is seen at the entrance of the ER area at Holy Cross Hospital, amid an outbreak of coronavirus disease (COVID-19), in Fort Lauderdale, Florida, U.S., April 20, 2020. REUTERS/Marco Bello/File Photo
The Biden Administration's employer COVID-19 vaccine rule should remain blocked because U.S. employers should not have to scramble to implement an illegal rule, opponents told a federal appeals court on Tuesday.
The U.S. 5th Circuit Court of Appeals in New Orleans is weighing whether to lift an order issued Saturday that froze the rule during litigation.
The government has been sued by private employers, religious organizations and states including Texas for allegedly exceeding its authority to issue the vaccine rule.
Businesses must begin crafting policies and collecting employee health information to prepare to implement the rule by the Jan. 4 deadline, while also dealing with labor shortages and logistics problems.
"These burdens start now," the opponents said in the court filing. "They cannot recover that lost time and effort if the rule is eventually enjoined."
The Biden Administration told the 5th U.S. Circuit Court on Monday that if it did not lift the stay on the rule, dozens or even hundreds of workers could die daily.
The opponents were also dismissive of the government's request that the court wait until next week, when other cases filed in federal appeals courts around the country will be grouped in one court.
The rule unveiled last week was issued by the Occupational Safety and Health Administration (OSHA) and mandates that businesses with at least 100 employees require staff get vaccinated against COVID-19 or be tested weekly and wear a mask.
President Joe Biden said in September, when COVID-19 cases were surging, that patience was wearing thin with unvaccinated Americans and he unveiled measures to boost inoculations.
Around two dozen states and many large companies have imposed their own vaccination requirements for employees. Around 80% of American adults have gotten at least one COVID-19 vaccine shot.
Retrieved from: https://www.reuters.com/world/us/opponents-urge-court-keep-bidens-covid-19-vaccine-rule-hold-2021-11-09/
By Emily Schmall and Hari Kumar

A woman received a Covid vaccine shot in Mumbai, India, last month. India drastically ramped up its vaccination drive to ease an earlier devastating surge.Credit...Divyakant Solanki/EPA, via Shutterstock
India’s coronavirus crisis was killing thousands of people a dayseven months ago. Now, as the nation celebrates the delivery of its one billionth vaccine dose, public health experts are sounding a new warning: The turnaround is losing steam.
Vaccinations are slowing down. As the temperature dips amid India’s most important festival season, people are crowding markets and hosting unmasked friends and family indoors. And the government is telling vaccination campaign volunteers like Namanjaya Khobragade that they are no longer needed.
“Now is not the time to let our guard down,” said Ms. Khobragade, a coordinator for a health nonprofit group in the eastern state of Jharkhand.
India’s progress, which represents a significant step toward ending the crisis globally, is an important political win for Prime Minister Narendra Modi, whose government came under heavy criticism for failing to prepare for a devastating second wave. After the virus killed tens of thousands of people, India’s government threw money at bolstering vaccine production, stopped vaccine exports and tossed out cumbersome inoculation rules.
By official figures, daily infections have plunged to about 12,000 per day from about 42,000 four months ago. Deaths, too, have fallen by half, to about 400 per day, though experts consider India’s statistics on infections and deaths to be a gross undercount.
But with only one-quarter of its vast population fully vaccinated, India is deeply vulnerable.
Retrieved from: https://www.nytimes.com/live/2021/11/09/world/us-travel-restrictions-ban-covid-19/india-billion-vaccine-doses
By Vjosa Isai

A health care worker administers the Pfizer-BioNTech vaccine at a pop-up clinic in Toronto this year.Credit...Carlos Osorio/Reuters
Canada’s health agency authorized booster shots of the Pfizer-BioNTech vaccine nationwide on Tuesday, broadening eligibility to anyone over the age of 18, regardless of what vaccine they received initially.
Health Canada, the federal department responsible for approving drugs, and the National Advisory Committee on Immunization had previously updated vaccine guidelines in September to recommend booster shots for seniors living in congregate settings and for people with compromised immune systems.
The new guidelines cite early evidence from two studies in Israel, including an Oct. 7 article published in The New England Journal of Medicine, which found that rates of “severe illness were substantially lower” for those who received a third Pfizer-BioNTech dose. Israel approved those booster shots on July 30.
In Canada, where the administration of health care falls under provincial control, some provinces had already begun to offer booster doses based on the interim federal guidelines. On Oct. 29, eligibility was expanded to frontline health workers, adults over 70, First Nations communities, and people who received two doses of AstraZeneca or one dose of the Johnson & Johnson vaccine.
Provinces including Ontario, British Columbia and Saskatchewan have already begun administering booster shots to these populations, or to people traveling to countries that require certain vaccines.
The Health Canada announcement on Tuesday standardized eligibility criteria across the country. The agency recommended that adults receive the Pfizer booster at least six months after their last dose.
Pfizer is also seeking regulatory approval for its vaccine to be administered to children aged 5 to 11, Prime Minister Justin Trudeau announced last month, with the country slated to receive 2.9 million pediatric doses when the authorization is granted.
As of Oct. 30, close to 74 percent of the country’s population was fully vaccinated, according to federal data.
Retrieved from: https://www.nytimes.com/2021/11/09/world/americas/canada-pfizer-booster-adults.html
Sajid Javid, Britain’s health secretary, said frontline health workers in England must be vaccinated against Covid-19 by April in order to “avoid preventable harm and protect patients.”
All frontline health workers in England must be vaccinated against Covid-19 by next spring to keep their jobs, Britain’s health secretary said on Tuesday, a move that employers and trade unions warned could aggravate staff shortages.
“We must avoid preventable harm and protect patients in the N.H.S., protect colleagues in the N.H.S. and, of course, protect the N.H.S. itself,” Sajid Javid, the health secretary, told Parliament, referring to the National Health Service. He added that about 90 percent of the service’s workers had received at least two vaccine doses.
The measure, which is subject to parliamentary approval, is due to come into force on April 1. Exemptions will be available for people who are medically prevented from receiving vaccines and for health workers who have no face-to-face contact with patients.
That time frame is intended to ensure that workers who do not want to be vaccinated remain in their jobs during the winter, when the strain on the country’s overstretched health service is likely to be particularly acute.
England’s health service employs around 1.3 million workers, though not all are in frontline positions. About 80,000 to 100,000 N.H.S. workers in the country remain unvaccinated against Covid, according to Chris Hopson, the chief executive of N.H.S. Providers, a membership organization for the N.H.S. hospital, mental health, community and ambulance services.
Scotland, Wales and Northern Ireland can make their own decisions on the issue, and so far have not put forward proposals.
In Britain, one main concern is that people reluctant to be vaccinated will quit their jobs and worsen staff shortages within a health service that is under acute strain and that expects more pressure as winter sets in.
“The problem for both social care and the N.H.S. is that we run these systems incredibly hot on very, very fine margins,” he told the BBC. “Both of us have got around 90,000 to 100,000 vacancies.”
As virus cases rise across Europe, President Emmanuel Macron of France announced that people over 65 years old must get a booster shot to remain eligible for a vaccine “passport,” which is required to gain access to restaurants, museums, long-distance trains and other public places. The new rule takes effect Dec. 15.
Roughly 308 million pieces of personal protective equipment bought by the Dutch government, including masks, goggles and gloves, were rejected for use in hospitals, a spokesman for the health ministry said. The Netherlands recently reintroduced measures to slow the spread of the virus and ease the growing pressure on the country’s health system. Last week almost 77,000 people tested positive for the virus, according to government figures.
Hong Kong’s latest “zero Covid” policy — a mandatory smartphone tracking app — is prompting online mockery and pushback. Residents must show their LeaveHomeSafe app to enter any public government facilities — including outdoor markets, libraries and pools. One nongovernmental organization warned that the requirement, which started on Nov. 1, would hurt the homeless and others who do not have a smartphone but depend on government services.
About 3,000 people marched through Wellington, New Zealand’s capital, on Tuesday to protest vaccination requirements and coronavirus restrictions, forcing a lockdown of Parliament and closure of local streets. In recent weeks the government has announced sweeping vaccine mandates for about 40 percent of the country’s workers, as well as requirements for vaccination certificates to gain access to restaurants, gyms and public events.
Retrieved from: https://www.nytimes.com/2021/11/09/world/europe/england-health-workers-vaccine-mandate.html
Here’s a round-up of the day’s leading Covid stories:
· France pushes to accelerate update of Covid-19 booster shots for elderly and vulnerable citizens. President Emmanuel Macron said a third injection would be made available to those aged 50-64 from early December. Anyone over 65 who was vaccinated more than six months ago will need to get a booster shot by mid-December for their “health pass” to remain valid, Macron said.
· Covid cases surge in Greece with a record of 8,613 new cases in the last 24 hours - the highest since the pandemic began. Cases have more than doubled in less than a fortnight.
· Virus deaths in Russia hit daily record of 1,211 Covid-19 deaths, the highest daily death toll in the pandemic, and 39,160 new cases. Russian president Vladimir Putin ordered many Russians last month to stay off work between 30 October and 7 November.
· Latvia, one of the least vaccinated countries in the European Union, is facing its most severe outbreak of Covid-19 yet.
· The World Health Organization has warned there could be shortfall of up to two billion syringes in 2022, which threatens to hamper vaccine efforts globally is production does not improve, AFP reports.
· Loved ones reunite at US-Mexico border as Covid travel restrictions lifted.
· UK health secretary says staff must be fully jabbed by April 2022 or risk dismissal raising concerns 32,000 care home staff and tens of thousands of NHS workers could quit.
· Daily Covid-related deaths in the UK rose above 250 again, with 262 reported on Tuesday.
· Covid-19 patients in Singapore who remain unvaccinated by choice will have to pay for their hospitalisation bills from 8 December, the government has ruled.
· Pfizer asks FDA to approve Covid booster shots for all US adults. Older Americans and other vulnerable groups have had access to a third dose of the Pfizer and BioNTech vaccine since September but the Food and Drug Administration has said it would move quickly to expand boosters to younger ages if warranted.
· Moderna also applied for European authorisation of its Covid-19 vaccine in children aged 6-11 years, weeks after it delayed a similar filing with US regulators.
· The European Union drugs regulator is set to authorise the use of two monoclonal antibodies to treat Covid-19 patients in coming days, two EU sources told Reuters, in its first approvals of such therapies.
· The US will buy another $1 billion worth of the Covid-19 pill made by Merck & Co Inc and partner Ridgeback Biotherapeutics, the companies said on Tuesday.
· Canada authorised the use of Pfizer/BioNTech’s vaccine as a booster shot for people 18 years of age and older.