
 i_need_contribute
i_need_contribute

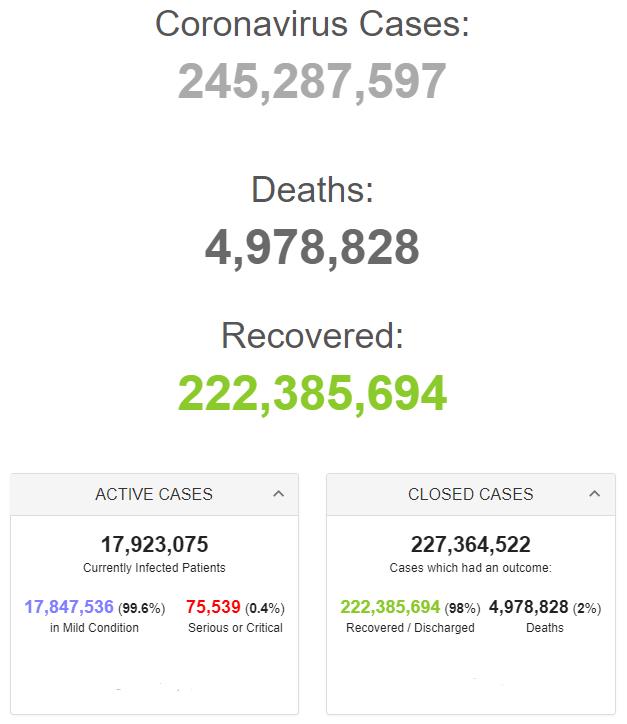
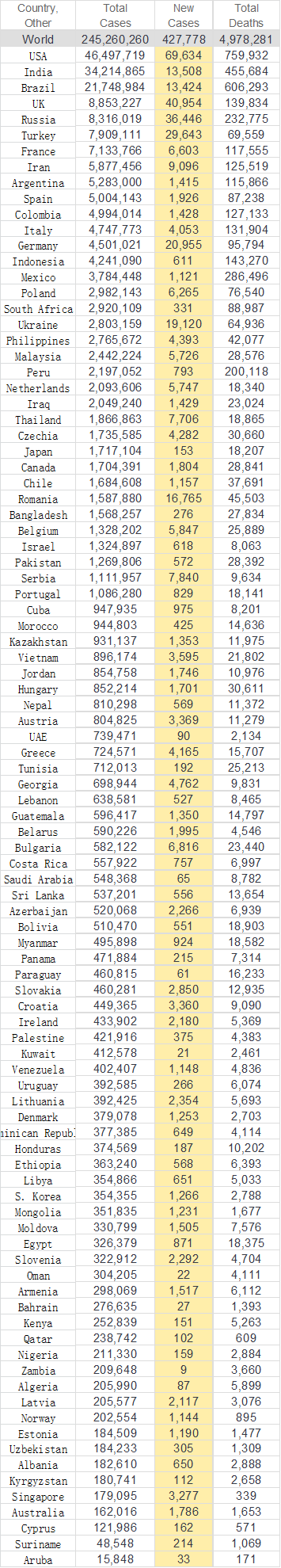
Retrieved from: https://www.worldometers.info/coronavirus/
By Manas Mishra and Michael Erman
An expert panel on Tuesday voted overwhelmingly to recommend the U.S. Food and Drug Administration authorize the Pfizer Inc (PFE.N) and BioNTech SE COVID-19 vaccine for children ages 5 to 11, saying the benefits of the shot outweigh the risks.
An authorization for that age group would be would be an important regulatory step toward reaching about 28 million children for inoculation, most of them back in school for in-person learning.
The vaccine could be available to the younger age group as soon as next week. The FDA is not obligated to follow the advice of its outside experts, but usually does. The vote was 17 in favor with one abstention.
If the FDA authorizes the shots for this age group, an advisory panel to the U.S. Centers for Disease Control and Prevention will meet next week to make a recommendation on the administration of the vaccine. The CDC director will make the final call.
While children becoming seriously ill or dying from COVID-19 is relatively rare compared with adults, some develop complications, and infections in unvaccinated kids have risen due to the easily transmitted Delta variant of the coronavirus. Data from the American Academy of Pediatrics shows that more than 500 U.S. children have died from COVID-19.
It "is the eighth highest killer of kids in this age group over the past year," said Dr. Amanda Cohn, a pediatric vaccine expert at the CDC and a voting member of the panel. "Use of this vaccine will prevent deaths, will prevent ICU admissions and will prevent significant long-term adverse outcomes in children."
Only a few other countries, including China, Cuba and the United Arab Emirates, have so far cleared COVID-19 vaccines for children in this age group and younger.
In the United States, just 57% of the population is fully vaccinated, lagging other nations such as the UK and France.
Still, the percentage of young children who receive the shots may be low. The U.S. vaccination rate for 12- to 15-year-olds trails other age groups at roughly 47%.
The World Health Organization since May has been urging rich countries to reconsider plans to vaccinate children and instead donate COVID-19 shots to the COVAX program for distribution to poorer countries.
A vial labelled with the Pfizer-BioNTech coronavirus disease (COVID-19) vaccine is seen in this illustration picture taken March 19, 2021. REUTERS/Dado Ruvic
LOWER DOSE
Pfizer and BioNTech are seeking clearance for a lower, 10-microgram dose of the vaccine in young children, versus 30 micrograms for those age 12 and older. The shot has been authorized for ages 12-15 since May after being cleared for those age 16 and older in December.
The companies have said their vaccine showed 90.7% efficacy against the coronavirus in a clinical trial of children aged 5 to 11.
The advisers paid close attention to the rate of a heart inflammation called myocarditis that has been linked to both the Pfizer/BioNTech and Moderna (MRNA.O) vaccines, particularly in young men.
If the number of myocarditis cases in the younger age group turns out to be similar to that in 12- to 15-year-olds, the hospitalizations prevented for COVID-19 would outnumber those prevented for myocarditis in most scenarios analyzed, FDA staff reviewers said in documents prepared ahead of Tuesday's meeting.
Some panel members suggested that due to the myocarditis risk, the vaccine should be given to a narrower group of children, such as those with conditions that make them more likely to be hospitalized.
"There are certain kids that should be vaccinated. The question of how broadly to use it, I think it's a substantial one," said Eric Rubin, Editor-in-Chief of the New England Journal of Medicine.
If authorized, the Pfizer/BioNTech vaccine is likely to be the only one available to the age group in the United States for some time.
On Monday, Moderna released data from its own clinical trial of children ages 6 to 11, and said it would soon ask regulators for authorization in the age group.
It is unclear when U.S. regulators will consider that. The company is still waiting for a response to its application filed in June for use of its vaccine in children ages 12 through 17.
Pfizer has said it could have data from its clinical trial in even younger children - ages 2 to 4 - by year end.
Retrieved from: https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-advisers-weigh-pfizerbiontech-covid-19-vaccine-children-2021-10-26/
By Renju Jose

A passenger walks with their luggage towards a Qantas Airways plane at Sydney International Airport in Australia, October 25, 2017. Picture taken October 25, 2017. REUTERS/Steven Saphore
All fully-vaccinated Australian citizens and permanent residents will be able to leave the country without a special exemption from Nov. 1, authorities said on Wednesday, as Australia eases coronavirus restrictions amid a rise in vaccination rates.
Australians have been unable to travel abroad for more than 18 months without a government waiver, while thousands of fully-vaccinated residents living abroad have been unable to return due to a cap on arrivals to slow the spread of COVID-19.
Many of these are now expected to return after Sydney and Melbourne ended quarantine rules for inoculated travelers from Nov. 1. Other cities, mostly virus-free, are expected to ease their border rules once they reach higher vaccination rates.
"The national plan is working ... (it) is about opening Australia up and that is because the vaccination rates are climbing so high," Prime Minister Scott Morrison told Seven News on Wednesday.
Australia's drug regulator, meanwhile, provisionally approved a booster dose of Pfizer Inc's (PFE.N) COVID-19 vaccine for people aged over 18, as first-dose vaccination levels in people over 16 neared 90%.
Federal Health Minister Greg Hunt said the rollout is expected to begin by Nov. 8 once the government receives advice from the country's vaccination technical advisory group.
The decision to lift the travel ban from next week comes after Singapore on Tuesday said it would allow quarantine-free entry to travellers vaccinated against COVID-19 from Australia from Nov. 8.
A third wave of infections fuelled by the Delta variant forced lockdowns in Australia's biggest cities, Sydney and Melbourne, and both have been gradually easing restrictions after racing through their vaccination targets.
Even with the Delta outbreaks, Australia has fared better than many comparable countries, with around 164,000 cases and 1,669 deaths. Victoria state reported 1,534 new cases on Wednesday, up from 1,510 a day earlier, while those in New South Wales rose to 304 from 282.
Retrieved from: https://www.reuters.com/business/healthcare-pharmaceuticals/australia-drugs-regulator-approves-booster-doses-pfizer-covid-19-vaccine-2021-10-26/
By Rebecca Robbins and Lynsey Chutel

A vaccination center in Dakar, Senegal, in July.Credit...Zohra Bensemra/Reuters
Facing pressure for keeping its Covid vaccine out of reach of poorer countries, Moderna said on Tuesday that it had agreed to sell up to 110 million shots to African Union member nations.
The company said it would deliver 15 million of the shots by the end of this year and 35 million more by the end of March, offering a modest supply boost for a continent with severe vaccine shortages and some of the world’s lowest vaccination rates.
The New York Times reported this month that Moderna’s shots have gone almost entirely to wealthier countries. Moderna has shipped a larger share of its doses to high-income countries than any other vaccine manufacturer, according to recent data from the data firm Airfinity.
Moderna also said on Tuesday that it was “working on plans” to bottle doses of its Covid vaccine somewhere on the African continent as soon as 2023, in addition to its plans announced this month to open a factory in Africa at an unspecified date.
Also on Tuesday, BioNTech — the German company that partnered with Pfizer on their Covid shot — said it planned in the middle of next year to start building a factory somewhere in Africa to manufacture vaccines that use mRNA technology. BioNTech also said it is in talks with Biovac, a South African manufacturer, about expanding an agreement under which Biovac has been contracted to start bottling the Pfizer vaccine.
Moderna has been sharply criticized for not sharing its vaccine recipe or transferring its technology to manufacturers in poorer countries that could make its shots for local markets.
Fewer than 6 percent of Africans are fully vaccinated against the coronavirus, and fewer than a third of African nations had fully vaccinated 10 percent of their populations by the start of this month.
“It’s a drop in the ocean for what the needs are,” Fatima Hassan, the head of the Health Justice Initiative in South Africa, said of Moderna’s announcement. “It’s up to 110 million for a population and a continent of 1.3 billion,” she said.
With the new deal with Moderna, the African Union now has two direct vaccine supply deals for its member countries. The African Union has ordered 220 million doses of Johnson & Johnson’s single-shot vaccine, with the option to order 180 million more. Deliveries from that order began in August.
Moderna and the African Union were in talks this past spring about a potential supply deal, but those talks fell apart because Moderna could not offer the doses until next year, according to two African Union officials. The negotiations restarted this month.
The company has repeatedly said that it is unable to supply more doses quickly to countries in need because it has limited manufacturing capacity and because all of its production this year had been locked up through existing orders from governments like the United States and the European Union.
Two officials in the Biden administration said the United States had agreed for some of its Moderna doses to be delivered several months later than planned so that Moderna could first supply the 15 million doses to the African Union. Moderna’s chief executive, Stéphane Bancel, said in a news release that the Biden administration had helped broker the deal.
Talks are continuing about Moderna potentially supplying more shots to poorer countries through other channels, one of the administration officials said.
Moderna did not say how much it was charging for the deal with the African Union, but two people involved in the negotiations said the deal was for doses at $7 per shot. By comparison, the United States has paid $15 to $16.50 for each shot, on top of the $1.3 billion the government gave Moderna to develop its vaccine. Several middle-income countries, including Botswana, have agreed to deals for $27 to $30 per Moderna shot.
Moderna has also agreed to sell more than 210 million doses, at an average purchase price of just under $10, to Covax, the United Nations-backed program to vaccinate the world’s poor. The company has not yet supplied any of those shots, a Covax spokesman said on Tuesday.
The tens of millions of Moderna doses that have made it to low- and lower-middle-income countries have been almost exclusively through donations from the United States. Those doses were distributed by Covax.
Retrieved from: https://www.nytimes.com/2021/10/26/world/africa/covid-moderna-african-union.html
By Oscar Lopez

Mexican students returned to classrooms at the end of August after a year of online learning.Credit...Jose Luis Gonzalez/Reuters
MEXICO CITY — As U.S. officials prepare to expand Covid vaccine eligibility to children ages 5 to 11, the Mexican government has resisted calls to vaccinate youths, despite a court order that it do so.
This month a judge ordered Mexico’s government to vaccinate anyone aged 12 to 17 after the parents of a 15-year-old girl sued to get their daughter vaccinated, just one of many lawsuits from parents demanding that their children be inoculated.
But President Andrés Manuel López Obrador dismissed the ruling as “not definitive” and hinted at challenging the decision, saying during a news conference that “legally this is going to be respected, but at the same time, we are going to go to the relevant authority to clarify” the court’s decision.
Whether the government would mount such a challenge remains unclear, but the president’s rhetoric is emblematic of Mexico’s continued resistance to allow minors to be inoculated, even as regulators in the United States and other countries have increasingly approved shots for children.
Mexico’s medical safety agency has granted the Pfizer-BioNTech vaccine emergency use authorization for youths 12 and over, but the government has refused to allow the shots to be administered to most minors, and has played down the risks Covid-19 poses to children.
Mexico has fully vaccinated only about 41 percent of its population, according to Our World in Data. The government has said it should concentrate on vaccinating the millions of adults who have yet to get shots, and put off vaccinating otherwise healthy children until the vaccines are proved to be safe for them.
The stance has been criticized by public health and political experts. Some say the government’s resistance to vaccinating children stems from a lack of planning and insufficient vaccine supplies.
“This mess comes from the lack of preparation,” said Xavier Tello, a public health policy expert in Mexico City, adding that the government has “no strategy.”
Hundreds of parents have taken the government to court and demanded shots for their children, and many have succeeded.
In the wake of the mounting media and legal pressure, Mr. López Obrador’s government said last month that it would begin vaccinating children over 12 who had an underlying condition, which could mean that more than one million are now eligible. But the government is holding firm on its commitment to vaccinate adults first.
“There is a vaccination plan,” Mr. López Obrador said this month, regarding the recent ruling mandating vaccines for teenagers. “A public policy cannot be defined based on the interest of a person or a group.”
Retrieved from: https://www.nytimes.com/live/2021/10/26/world/covid-vaccine-boosters/covid-mexico-vaccine-children

The inaugural session of Germany’s lower house of Parliament in Berlin on Tuesday.Credit...Fabrizio Bensch/Reuters
How can you tell whether German lawmakers have some protection against the coronavirus or have tested negative?
Look at their wrists.
As the country’s newly elected federal lawmakers met in the main hall of the historic Reichstag building on Tuesday, they were doing so for the first time in a year and a half in full numbers and without social distancing.
To ensure the lawmakers’ safety, those taking part were required to show proof of vaccination, recent infection or a negative test result. And so they don’t have to show their documents multiple times, they were given wristbands of red, black and gold to indicate that their status had been checked.
Over 700 of Germany’s federal lawmakers, elected to represent their constituents a month ago, were meeting to choose a new parliamentary president. Chancellor Angela Merkel and her cabinet remain in their posts until a new government is formed.
Lawmakers not wanting to show their papers were allowed to follow the first meeting in reserved and socially distanced seats in the visitors’ balcony.
Ms. Merkel, who did not run for re-election in the recent vote and is therefore no longer a member of Parliament, watched the proceedings from the balcony.
Retrieved from: https://www.nytimes.com/live/2021/10/26/world/covid-vaccine-boosters/in-germanys-parliament-wristbands-indicate-lawmakers-covid-status
By Jessica Elgot and Linda Geddes
Pregnant women are being turned away from Covid vaccine clinics despite clinical advice, experts have warned as they urged ministers to ramp up efforts to reach unvaccinated groups.
Members of the Joint Committee on Vaccination and Immunisation (JCVI) told the Guardian that efforts to increase booster jab uptake will not be sufficient to prevent more deaths and hospitalisations, and that ministers must prioritise reaching those who have had no jabs. In particular they urged a focus on pregnant women as only about 15% in the UK have been fully vaccinated. Among all over-12s, the figure is 79%.
On Tuesday the NHS said pregnant women should never be turned away from clinics and said vaccines could save the lives of women and their babies.
New data from Oxford University’s MBRRACE-UK study on maternal health, seen by the Guardian, shows that at least 13 pregnant women died with Covid between July and September this year, with 85% of them believed to have been unvaccinated. The figure is higher than in the first and second waves of the pandemic, when nine and 11 pregnant women died but when jabs were not available.
Prof Marian Knight, the lead for the MBRRACE-UK programme, said there was still no joined-up messaging across the health service. “Women are being turned away from clinics and now there are some trusts offering it as part of the maternity service, but it is not universal so there are still barriers,” she said.
“It is important we start to see data on outcomes in vaccinated women so we can show evidence that vaccines are safe, rather than say there’s no evidence they cause harm. These are very small numbers, but the point is that women could have been saved; children have been orphaned.”
Joeli Brearley, the founder of the charity Pregnant Then Screwed, said old leaflets advising against the jab were still in circulation at some healthcare centres. Some professionals were giving conflicting advice on safety, with hesitancy also driven by the fact that pregnant women were not yet included in the booster jab drive, she added. “It sends out the message: we are not sure about pregnant women and the vaccination.”
Covid in pregnancy is linked to a raised risk of premature delivery, while pregnant women are more likely to become seriously ill than non-pregnant women of the same age. A previous study found that one in six of 118 Covid patients requiring the most intensive ventilation treatment were unvaccinated pregnant women.
Other research suggests the Delta variant poses a greater threat to pregnant women than previous variants. In July the UK Obstetric Surveillance Systemfound that one in four pregnant women admitted to hospital with Covid in the first wave had moderate to severe disease compared with 45% of recent admissions. Between March and July, one in three pregnant women in hospital with Covid required respiratory support, and one in seven required intensive care.
Pregnant women are among those that some members of the JCVI, which advises the government on vaccine policy, are most concerned about. However, some scientists have privately accused the JCVI itself of being slow to advise that the vaccine was safe for pregnant women.
An NHS spokesperson said: “Pregnant women should not be turned away from NHS vaccination centres and women should continue to come forward for the lifesaving Covid vaccine – they can make a booking through the national booking service online or by calling 119 anytime between 7am and 11pm seven days a week.
“The NHS has advised midwifery staff to give pregnant women the information they need to make the right decision for them and their baby so if you are pregnant and have any concerns, please come forward and discuss them with a healthcare professional.”
Adam Finn, a professor of paediatrics at the University of Bristol and a member of the JCVI, said: “It does now seem clear that not only does Covid in pregnancy bring an increased risk of premature delivery but also pregnant women are more likely to get seriously ill than non-pregnant women of the same age. So there’s a need to communicate that effectively and that can probably most effectively be done by midwives.
“The people who benefit most from vaccines, whether or not they are pregnant, are those who have had no doses so far. Explaining to people why this makes sense and is important for them is, perhaps, the most worthwhile thing we could be doing at present.”
Another member of the JCVI, Maggie Wearmouth, said: “The message is just not reaching many pregnant women … Saving lives and reducing admissions needs more active outreach to the 4 million people not yet vaccinated at all, particularly pregnant women and young black men.”
Amid criticism of the pace of the booster rollout, the government has pledged to intensify efforts to improve take-up of the third jab offered to those over 50 and the clinically vulnerable. Last week, government sources said they would examine whether to cut the period that must elapse before a booster jab from six months to five.
Members of the JCVI are understood to be sceptical about the effects of such a move. Finn said: “Getting the booster programme done as quickly as possible is only one aspect of a complex situation.
“It’s important to immunise the right people – those who actually need a booster – at the right time, when their response to it will be substantial and as long-lasting as possible. It’s also important not to overestimate what the booster programme is capable of – after all it is simply increasing the level of protection against serious illness in people whose protection from the first two doses is still pretty good.
“Finally, the booster programme will not do much to reduce the circulation of the virus more generally or any time soon – other precautions would be needed to achieve that.”
Wearmouth said it was highly unlikely there would be any clinical benefit to speeding up the booster jabs. “The sooner a booster is given, the sooner it may have to be given yet again,” she said. “We are clearly losing the battle by depending on vaccinations alone. It is time for a serious review of wider aspects. We need politicians and known faces to be seen to have their boosters as well as observing social distancing, masks, etc.”
Prof Anthony Harnden, deputy chairman of the JCVI, told Sky News: “Vaccines do a lot of the heavy lifting, but they can’t do everything, so social distancing, mask wearing in crowded spaces and being sensible is all part of what we ought to be doing as a society.”
Retrieved from: https://www.theguardian.com/world/2021/oct/26/pregnant-women-are-being-turned-away-from-covid-vaccine-clinics-experts-warn
Here’s a round-up of the day’s leading Covid stories:
· The Covid-19 crisis is far “far from finished”, the World Health Organization’s emergency committee has said. The 19-member committee, which meets every three months to discuss the pandemic and make recommendations, also called for research into next-generation vaccines and long-term action to control the virus.
· Vaccine booster rates are now exceeding first-shot rates across the US, according to data from the Centers for Disease Control and Prevention (CDC).
· In Thailand, businesses are pleading with the government to drop the nation’s current alcohol ban when the country reopens, saying it will deter tourists.
· China has locked down a city of 4m over 6 Covid cases. Residents in Lanzhou, Gansu, have been told to stay at home as buses, taxis and key rail routes are suspended.
· FDA advisers recommend approval of Pfizer’s Covid vaccine for children aged 5-11. it will be the first vaccine available for younger children in the US. The nearly unanimous vote clears the way for possible approval for emergency use next month, making nearly 30m children eligible.
· Pregnant women are being turned away from Covid vaccine clinics despite clinical advice, experts have warned as they urged ministers to ramp up efforts to reach unvaccinated groups.
· The UK recorded 40,954 new Covid cases today and 263 more people have died, official figures show.
· A Brazilian Senate committee recommended on Tuesday that president Jair Bolsonaro face a series of criminal indictments for actions and omissions related to the world’s second highest Covid-19 death toll. The 7-to-4 vote was the culmination of a six-month committee investigation of the government’s handling of the pandemic.
· No exemptions are to be given for unvaccinated tennis players travelling from overseas for the Australian Open, the state’s premier has said. Players like Novak Djokovic has repeatedly refused to reveal his vaccination status.
· Fully vaccinated Australians will no longer have to apply for travel exemptions to leave the country, as Australia prepares to ease its international borders from 1 November.
· Australia could hit the 80 per cent full Covid-19 vaccination mark within a week.
· Russia, Bulgaria and the Ukraine all reported a record number of daily deaths on Tuesday. Russia reported 1,106 deaths in 24 hours, the most since the start of the pandemic bringing the total death toll to 232,775, Europe’s highest by far. Sluggish vaccination rates have allowed the virus to spread quickly across Eastern Europe.
· Georgia Republican Marjorie Taylor Greene has been fined for the third time for refusing to wear mask on House floor.