
 i_need_contribute
i_need_contribute

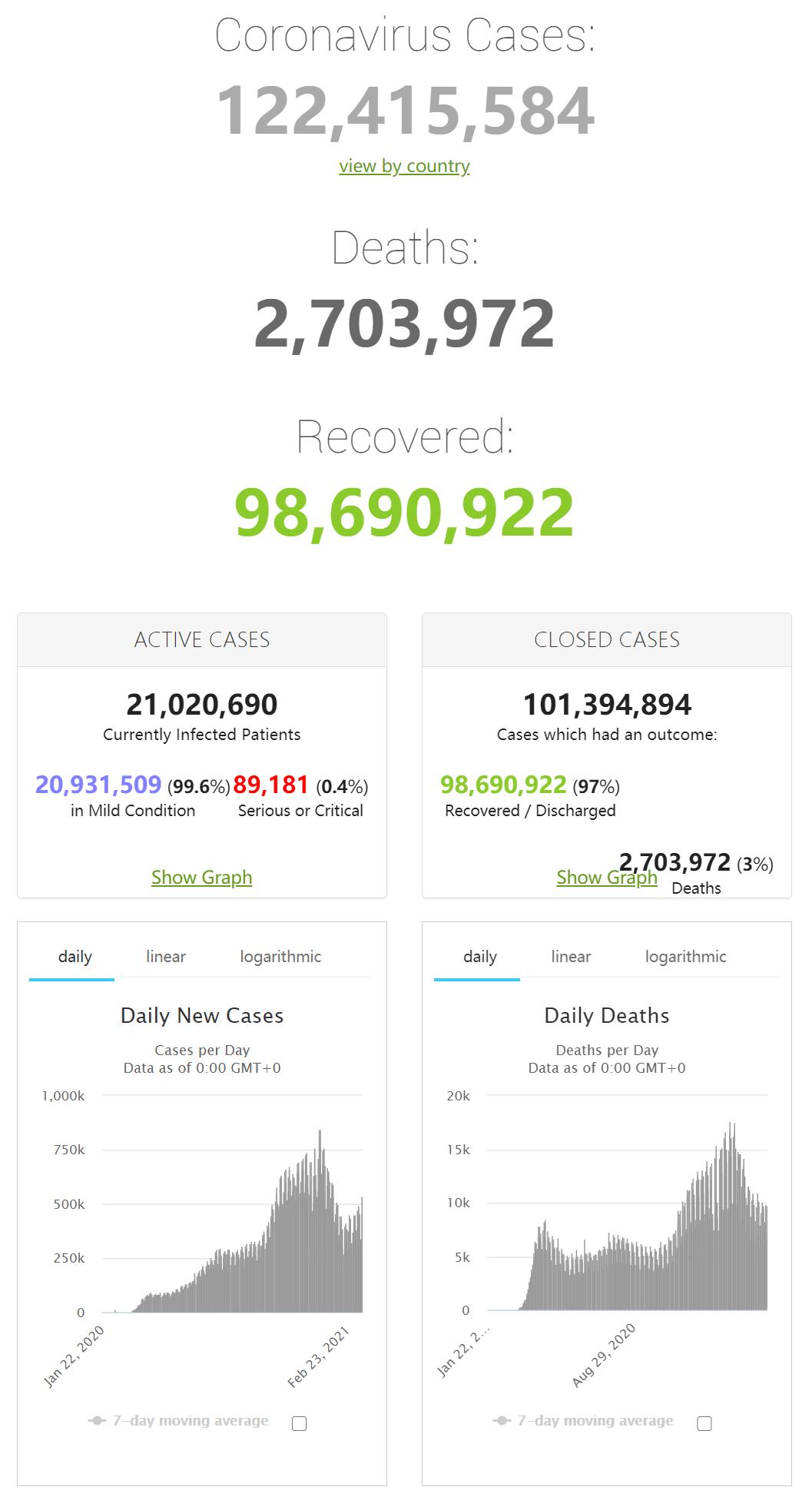
| Country, | Total | New | Total |
| Other | Cases | Cases | Deaths |
| World | 122,358,105 | 546,090 | 2,702,463 |
| Brazil | 11,787,600 | 87,169 | 287,795 |
| India | 11,513,945 | 39,643 | 159,405 |
| Russia | 4,428,239 | 9,803 | 93,824 |
| UK | 4,280,882 | 6,303 | 125,926 |
| France | 4,181,607 | 34,998 | 91,679 |
| Italy | 3,306,711 | 24,935 | 103,855 |
| Spain | 3,212,332 | 6,216 | 72,910 |
| Turkey | 2,950,603 | 20,049 | 29,777 |
| Germany | 2,628,629 | 17,860 | 74,878 |
| Colombia | 2,319,293 | 5,139 | 61,636 |
| Argentina | 2,226,753 | 8,328 | 54,386 |
| Mexico | 2,175,462 | 6,455 | 195,908 |
| Poland | 1,984,248 | 27,278 | 48,388 |
| Iran | 1,778,645 | 7,530 | 61,581 |
| South Africa | 1,533,961 | 1,464 | 51,724 |
| Ukraine | 1,504,076 | 15,053 | 29,253 |
| Indonesia | 1,443,853 | 6,570 | 39,142 |
| Peru | 1,443,521 | 7,923 | 49,706 |
| Czechia | 1,439,019 | 11,928 | 24,117 |
| Netherlands | 1,179,612 | 6,125 | 16,198 |
| Canada | 922,848 | 3,609 | 22,590 |
| Chile | 911,469 | 6,257 | 21,988 |
| Romania | 881,159 | 6,174 | 21,877 |
| Israel | 825,562 | 1,384 | 6,069 |
| Belgium | 818,142 | 5,116 | 22,600 |
| Portugal | 816,055 | 485 | 16,743 |
| Iraq | 779,458 | 5,443 | 13,896 |
| Sweden | 738,537 | 13,236 | |
| Philippines | 640,984 | 5,290 | 12,887 |
| Pakistan | 615,810 | 3,495 | 13,717 |
| Switzerland | 578,861 | 1,750 | 10,183 |
| Bangladesh | 564,939 | 2,187 | 8,624 |
| Hungary | 539,080 | 6,502 | 17,628 |
| Serbia | 536,904 | 5,346 | 4,840 |
| Jordan | 514,107 | 9,192 | 5,627 |
| Austria | 504,581 | 3,357 | 8,982 |
| Morocco | 490,575 | 487 | 8,748 |
| Japan | 451,186 | 1,473 | 8,717 |
| UAE | 434,465 | 2,101 | 1,424 |
| Lebanon | 430,734 | 3,757 | 5,609 |
| Saudi Arabia | 383,880 | 381 | 6,591 |
| Panama | 349,505 | 485 | 6,025 |
| Slovakia | 344,470 | 2,040 | 8,814 |
| Malaysia | 328,466 | 1,213 | 1,223 |
| Ecuador | 307,429 | 1,831 | 16,333 |
| Belarus | 306,524 | 1,254 | 2,130 |
| Bulgaria | 295,777 | 4,008 | 11,817 |
| Georgia | 276,436 | 369 | 3,674 |
| Nepal | 275,625 | 107 | 3,015 |
| Bolivia | 262,056 | 1,068 | 12,015 |
| Croatia | 254,507 | 1,197 | 5,726 |
| Dominican Republic | 247,979 | 615 | 3,257 |
| Tunisia | 243,935 | 496 | 8,490 |
| Azerbaijan | 243,424 | 933 | 3,314 |
| Greece | 230,317 | 3,070 | 7,297 |
| Ireland | 228,796 | 581 | 4,566 |
| Kazakhstan | 227,953 | 1,186 | 2,881 |
| Denmark | 223,415 | 786 | 2,397 |
| Palestine | 218,061 | 2,077 | 2,358 |
| Kuwait | 215,067 | 1,394 | 1,202 |
| Moldova | 210,725 | 1,797 | 4,472 |
| Costa Rica | 210,447 | 2,886 | |
| Lithuania | 207,469 | 552 | 3,442 |
| Slovenia | 203,544 | 972 | 3,951 |
| Egypt | 193,482 | 642 | 11,472 |
| Paraguay | 188,493 | 2,605 | 3,620 |
| Guatemala | 185,832 | 898 | 6,639 |
| Ethiopia | 181,869 | 2,057 | 2,602 |
| Armenia | 181,165 | 1,024 | 3,301 |
| Honduras | 180,271 | 756 | 4,390 |
| Qatar | 172,200 | 499 | 270 |
| Nigeria | 161,409 | 135 | 2,027 |
| Bosnia and Herzegovina | 149,891 | 1,728 | 5,729 |
| Libya | 149,207 | 1,032 | 2,435 |
| Oman | 149,135 | 577 | 1,620 |
| Venezuela | 148,208 | 631 | 1,467 |
| Myanmar | 142,212 | 22 | 3,204 |
| Bahrain | 133,779 | 642 | 491 |
| Albania | 119,528 | 590 | 2,106 |
| Kenya | 117,535 | 1,225 | 1,954 |
| North Macedonia | 116,438 | 1,216 | 3,403 |
| Algeria | 115,842 | 154 | 3,051 |
| S. Korea | 97,294 | 445 | 1,688 |
| Latvia | 95,902 | 482 | 1,801 |
| Estonia | 91,031 | 1,700 | 758 |
| China | 90,072 | 6 | 4,636 |
| Sri Lanka | 89,024 | 162 | 537 |
| Ghana | 88,421 | 705 | |
| Kyrgyzstan | 87,143 | 98 | 1,485 |
| Zambia | 85,889 | 387 | 1,175 |
| Montenegro | 85,774 | 521 | 1,178 |
| Norway | 84,553 | 1,034 | 648 |
| Uzbekistan | 80,971 | 113 | 622 |
| Uruguay | 76,816 | 1,678 | 749 |
| Finland | 69,497 | 804 | 805 |
| Mozambique | 65,452 | 255 | 737 |
| Cuba | 64,414 | 689 | 384 |
| El Salvador | 62,531 | 1,962 | |
| Singapore | 60,152 | 15 | 30 |
Retrieved from: https://www.worldometers.info/coronavirus/

A Johnson & Johnson technician works on a COVID vaccine candidate in Belgium on June 17, 2020. Olivier Matthys/Getty Images
Johnson & Johnson is working to develop modified versions of its COVID-19 vaccine that may be needed to protect against virus variants, a report said.
“We’re working on several next generations of vaccines,” J&J Chief Executive Alex Gorsky said Thursday during an online discussion, according to The Wall Street Journal.
Gorsky expressed optimism that its current vaccine could offer some protection against new COVID-19 strains.
But, he said, “We have to be prepared,” the newspaper reported. “We should prepare for the worst and hope for the best.”
Johnson & Johnson’s single-dose vaccine was approved by the US Food and Drug Administration in late February.
The FDA affirmed J&J’s finding that the vaccine was 66 percent effective overall at warding off moderate and severe COVID-19 cases and 85 percent effective at preventing the most serious infections.
https://nypost.com/2021/03/18/johnson-johnson-working-on-vaccines-for-covid-19-variants/

EPA
Some 1,200 people are said to be in intensive care in hospitals across Paris
The French capital Paris is set to go into a month-long Covid lockdown as the country fears a third wave.
Another 15 departments in the country will also be placed under the same measures from midnight on Friday.
These measures will not be as strict as the previous lockdown, Prime Minister Jean Castex said, with people allowed to exercise outdoors.
France has recorded more than 35,000 new infections within the past 24 hours.
Mr Castex said a "third wave" of infections in the country was looking increasingly likely.
The situation in Paris is particularly worrying with 1,200 people in intensive care there, more than at the peak of the second wave in November, Health Minister Olivier Veran said.
Under the new measures, non-essential businesses will be forced to close but schools will remain open. People will be allowed to exercise outdoors within 10km (6 miles) of their home and are not allowed to travel to other parts of the country unless they have a valid reason. Those in the affected areas will have to fill out a form to explain why they have left their homes.
France's nationwide curfew will remain in place. However, it will begin an hour later at 19:00 (18:00 GMT), taking into account the longer hours of daylight.
Fears of a third wave come as the French government faces criticism for its slow vaccine rollout.
From Friday, France will resume vaccinating using the AstraZeneca jab following the EMA's announcement that it was fit for use. Mr Castex said he would be getting the vaccine straight away to prove that it was okay.
France had suspended the jab after a number of cases in Europe of blood clots developing after the vaccine was administered. A survey conducted just as the suspension was announced found that only 20% of the French have confidence in AstraZeneca.
https://www.bbc.com/news/world-europe-56450880
The European Union’s drug regulator said on Thursday that the AstraZeneca vaccine was safe, a finding that officials hope will alleviate concerns about possible side effects and prompt more than a dozen countries to resume using it against the resurgent coronavirus.
The regulator, the European Medicines Agency, said a new warning label will be added to the shot so that people in the medical community can be on the lookout for a potential rare complication leading to bleeding in the brain.
Despite reports of a small number of cases of dangerous blood clots in people who had received the vaccine, a review of millions of cases found that it does not increase the overall risk of clots, though “there are still some uncertainties,” said Dr. Sabine Straus, who heads the agency’s risk assessment committee.
Last week and early this week, several European countries suspended use of the AstraZeneca vaccine, a pause that, however brief, threatens lingering consequences both on that continent, which is struggling to contain a new wave of infection, and around the world.
Europe is not inoculating people quickly enough to slow transmission of the virus, the World Health Organization said on Thursday, reporting that new infections had risen for three successive weeks and that more people in the region were dying from the disease than a year ago.
The AstraZeneca vaccine shot, more easily stored than Pfizer and Moderna, and sold for now without the goal of earning a profit, is a keystone of the W. H.O.’s effort to inoculate poor and middle-income countries.
“This is a safe and effective vaccine,” said Emer Cooke, the head of the European regulator.
A fearful public may not be easily reassured.
“I haven’t decided yet whether I am going to get vaccinated or not,” said Giada Pietrolillo, a kindergarten teacher in Calabria, at the southern tip of Italy. “I am not sure I trust anyone any more.”
While the vast majority of the roughly 20 million people in Europe who have received the AstraZeneca shot suffered no serious side effects, Dr. Straus said, there were a handful of troubling cases of cerebral venous thrombosis, blood clots in the brain that lead to hemorrhages, in patients who also had low platelet counts. The evidence, she said, is not conclusive as to whether it is related to the vaccine
The officials said they hoped a clear statement on the safety of the vaccine would calm anxious governments and their populations at a particularly precarious moment in the pandemic.
Manuela Perozzi, a teacher for handicapped pupils at a middle school in Campobasso, in southern Italy, said she was sick with fever and aches for two days after her first dose of the AstraZeneca shot earlier this month, and then grew worried as fears about it spread.
“We can only try to hold on to science,” she said. “But surely I will be even less serene when they call me for the second shot.”
The leaders of the nations, mostly in Europe, that paused its use earlier this week framed their decision as a move intended to reassure the public that all concerns were being treated seriously, adding that they would await guidance from the regulator. Most of the countries had signaled that they were likely to restart using the vaccine once the agency issued clearance.
The Italian government on Thursday welcomed the drug regulator’s findings and said it would end its suspension “as of tomorrow.” France also announced it would return to giving out the vaccine, and the prime minister, Jean Castex, said he himself would get a shot. Spain will resume its rollout next Wednesday, said Carolina Darias, Spain’s health minister. At the time of the suspension, about 930,000 Spaniards were waiting for a second dose of AstraZeneca’s vaccine.
Norway’s health authority said it would continue to study the issue before deciding whether to lift its suspension.
Despite their differences, all the vaccines approved by Western regulators have shown themselves to be remarkably effective at reducing severe illness and death. And though the AstraZeneca vaccine accounts for less than 20 percent of the hundreds of millions of doses ordered by the European Union, it was a critical part of early rollout plans.
With infections again on the rise in many European countries, the cost of delay may be measured in lives.
In just one week in January, at the height of the last wave, Europe recorded nearly 40,000 deaths.
This week, more people are on ventilators in hospitals in Poland than at any time in the pandemic, leading officials to reimpose national restrictions, starting on Saturday. Italy has reimposed lockdowns in the hopes of limiting outbreaks. Across the continent, there is rising concern about the spread of variants of the virus.
https://www.nytimes.com/live/2021/03/18/world/covid-19-coronavirus#us-vaccine-pace-eligible


Workers lined up on Tuesday to take coronavirus tests in the South Korean city of Ansan, south of Seoul.Credit...Yonhap/EPA, via Shutterstock
The authorities in Seoul, the South Korean capital, issued mixed messages on Thursday about a contentious plan to test all foreign workers in the city for the coronavirus, leading to criticisms that the proposal was xenophobic and discriminatory.
On Wednesday, the Seoul Metropolitan Government said that hundreds of thousands of foreigners in the city would be required to undergo testing after a spike in infections among foreign workers.
Officials said that all companies that employ at least one foreigner had 15 days from Wednesday to send their workers for testing or face fines of up to 2 million won, about $1,700.
The announcement was met with anger, and diplomats and Korean politicians called for the order to be revoked.
“The administrative order of the Seoul city government is an unfair racist act against foreigners, and it is so ridiculous,” Lee Sang-min, a lawmaker from the governing Democratic Party, wrote on Facebook. “It is a human rights violation that would disgrace South Korea internationally.”
But as some city officials insisted that the tests were mandatory, others indicated that they were recommended but not required.
An official in the city’s foreign-labor department said that although the city could not force foreign workers to take a test, those who did not get tested before the deadline could face financial penalties if they were later found to be infected. The penalties include paying for treatment for anyone they made sick.
The Seoul Metropolitan Government later walked back those claims and said all foreign workers in Seoul would be required to get a test, including unregistered foreign workers.
The mixed messages led to confusion, even as hundreds of workers flocked to designated testing sites across the city. The government said it could test up to 3,600 foreigners a day over the next two weeks.
Park Yoo-mi, a city quarantine officer, told reporters that a recent cluster among foreign workers had prompted the city to order the testing.
“The coronavirus cases of foreigners count 6.3 percent of entire cases in Seoul from January to March this year, and the number keeps increasing,” she said.
Last week, the authorities in Gyeonggi, the province that surrounds the capital, issued a similar order for foreign workers to undergo testing.
Graham Nelson, a political counselor at the British Embassy in Seoul, criticized the plan, likening discrimination to a disease.
“Both coronavirus and discrimination are fatal diseases,” he wrote on Twitter. “Many foreigners are expressing concerns on the movement of regions, including Gyeonggi province, Seoul city and South Jeolla province, requiring only foreigners for testing.”
https://www.nytimes.com/live/2021/03/18/world/covid-19-coronavirus?name=styln-coronavirus-live®ion=TOP_BANNER&block=storyline_menu_recirc&action=click&pgtype=LegacyCollection&impression_id=&variant=1_Show
The number of new daily infections is at its highest level since January 22, according to the latest figures from the Robert Koch Institute for infectious diseases.
Cases in Germany have been on the rise after gradually declining throughout the lockdown
Germany has recorded its highest number of new COVID-19 infections since January, according to data published by the Robert Koch Institute (RKI) for infectious diseases on Thursday.
There were 17,504 new cases of the virus recorded over the past 24 hours, up from 13,343 recorded on Wednesday. The rate of infection reached 90 new cases per 100,000 people over the past seven days.
Germany began to relax lockdown restrictions at the beginning of March. If the infection rate stays above 100 for three days in a row then an emergency brake will allow authorities to reimpose lockdown measures.
The RKI has said that Germany is now in a third wave of infections — made worse by the spread of more contagious variants of the virus — and predicted a big jump in cases.
Germany has been slammed for its faltering vaccination drive. Chancellor Angela Merkel said that all Germans will have been offered a vaccine by the end of September, but so far only 8.4% of the population — almost 7 million people — has received at least one dose of COVID-19 vaccine. The share of people who had been fully vaccinated was at 3.7%.
The German government's vaccine dashboard reported that 198,421 people were vaccinated on Wednesday.
The health ministry suspended the use of the AstraZeneca vaccine on Monday, citing reports of unusual blood clots among people who had received the vaccine — critics have said that the suspension threatens to slow down the national vaccination campaign.
https://www.dw.com/en/germany-reports-highest-daily-covid-cases-for-two-months/a-56912402
By Kylie Atwood, Kaitlan Collins and Betsy Klein, CNN
Washington (CNN)The Biden administration is finalizing plans to send millions of AstraZeneca Covid-19 vaccine doses stockpiled and waiting for official usage approval in the US over the border to Mexico and Canada, according to White House press secretary Jen Psaki.
"I can confirm that we have 7 million releasable doses available of AstraZeneca," Psaki said at Thursday's White House press briefing. "2.5 million of those, we are working to finalize plans to lend those to Mexico and 1.5 million to Canada," she added.
President Joe Biden could announce the agreement upon finalization, which could happen as soon as Friday, CNN has learned. On Tuesday, Mexico's foreign minister said an announcement could come by the end of the week.
"I'd say we've made good progress, but the details, figures, provisions, won't be known until Friday," Mexican Foreign Minister Marcelo Ebrard told reporters on Tuesday morning, according to Reuters. "We requested as many (AstraZeneca doses) as possible."
Biden has met virtually with both Canadian Prime Minister Justin Trudeau and Mexican President Andrés Manuel López Obrador. During the conversations, they both pressed him on the need for more vaccines. Mexican government officials also pressed Biden officials on helping with vaccine supply during conversations between both parties regarding the surge on the southern border, an administration official told CNN.
The Biden administration has committed to having enough vaccines for all Americans before sharing doses, and if this agreement comes together it would be the first time the US has shared vaccines directly with another country. It would also likely give a major boost to vaccination efforts in Canada and Mexico who are struggling with their vaccine roll-out in comparison to the US.
On Wednesday, White House press secretary Jen Psaki confirmed that requests have been received from both Mexico and Canada and said that they are being considered carefully. She provided no details on when a decision would be reached.
The other administration official told CNN that one option under consideration is a swap agreement with the two countries: an agreement to share the AstraZeneca doses now with the condition that Mexico and Canada will share excess vaccines with the US in the future.
There are tens of millions of AstraZeneca doses stockpiled in the US and the company believes it will have roughly 50 million Covid-19 vaccine doses available to the US government by the end of April. None of those doses are available to Americans now because AstraZeneca has not applied to the Food and Drug Administration for an emergency use authorization, and the vaccine is still going through clinical trials in the US.
AstraZeneca has been approved for use in both Canada and Mexico, and the company itself has asked the Biden administration to consider requests to donate inventory to other nations.
Mexican President Andres Manuel Lopez Obrador announced Monday that he was close to reaching two agreements on vaccines, but he didn't specify which countries would be sending them. Another top official in Mexico publicly asked the US to share AstraZeneca vaccines earlier this week.
A Canadian Embassy spokesperson said that there have been "great engagements" with the Biden administration about Covid-19 and added that "conversations are ongoing" when it comes to getting more Canadians vaccinated. The spokesperson did not comment on the possible swap agreement.
https://www.cnn.com/2021/03/17/politics/us-astrazeneca-mexico-canada/index.html